By PETERS. MEYERS PRESENTED AT THE 55TH ANNUAL INTERNATIONAL WATER CONFERENCE PITTSBURGH, PENNSYLVANIA, OCTOBER 31 - NOVEMBER 1-2, 1
This progress report started with some samples of resin used to soften oil field produced water. ResinTech was asked to evaluate various commercially available cleaners used for iron removal. The resin samples were fouled with oil, typical of softener resins used in this application. However, there was very little iron fouling on the resin. The cleaners designed to remove iron fouling did little to improve the softening performance. One cleaner, designed for oil fouling, followed by a cleaner designed for iron fouling, did produce a significant improvement in the softening capacity of the resin.
Further experimentation with various other cleaners traditionally used for oil fouling produced disappointing results.
Sodium Hypochlorite has occasionally been used as a resin cleaner. Low levels of chlorine for long periods of time damage ion exchange resin. There is a perception that high levels of bleach will damage resins in a short period of time.
We found that bleach is an effective resin cleaner for oil. Furthermore, we found that very little damage was done to the resin after short exposures to relatively high bleach concentrations.
Bleach is a relatively inexpensive and safe cleaner. Although some damage to the resin may occur, we believe that bleach should be considered for treating oil fouling and possibly for other types of organic fouling, particularly for heavy fouling where milder cleaners are not effective.
Introduction
It is no big secret that oil fouls ion exchange resin. Even at low concentrations, oil coats the surface of the beads and blocks the pores. The exchange of ions into and out of the resin beads is made more difficult due to the barrier created by the oil layer. At higher levels of oil fouling, the resin beads become sticky and clump together. This leads to a greater risk of resin loss during backwash. It can also lead to flow channeling with all the associated symptoms of poor performance.
Softeners that are used to soften oil field produced water are famous for becoming oil fouled. Oil fields produce water along with the oil that is pumped from the ground. This water is separated from the oil, treated, and then re-injected. In the thermal process for enhanced oil recovery, the produced water is softened before it is introduced into wet steam generators. There are hundreds of thousands of cubic feet of softening resins used in this application, worldwide.
Cation resins are reasonably good coalescing medias. Even when there is not any "free oil" in the feedwater, there is sufficient dissolved oil to foul the resin. The effect is low capacity and sometimes high hardness leakage. This is a chronic problem with softeners used in this application.
Although less common in industrial and utility installations, oil fouling can occur in any resin bed. Diaphragm metering pumps are well known to fail and pump hydraulic oil into resin beds. Air systems can fail to deliver oil free air for mixing or scrubbing. A variety of maladies in water supply and distribution systems can result in oil fouled resin.
Description of the column study procedure
Several 1-inch diameter resin columns were set up at ResinTech's laboratory and filled with approximately 100 ml each of the oil fouled resin "as received" from the customer.
Where the cleaner used was supplied as a premixed commercially available product, the manufacturer's cleaning procedure was followed. Where generic cleaners were used, ResinTech's procedures were used.
The effectiveness of each cleaner was evaluated by measuring the regeneration efficiency after cleaning and comparing it to that of a resin sample that was not cleaned and to that of new resin. Many cleaning procedures include single or double regenerations before and after cleaning. The extra chemical dose can produce an apparent performance improvement that is not attributable to the cleaner itself.
By deliberately exhausting the resins after cleaning, this variable is excluded from the results. Regeneration efficiency is a measure of a resin's operating capacity as a function of the chemical dose and therefore is a true reflection of the resin's performance.
After cleaning, the resin samples were exhausted into the calcium form using an excess of calcium chloride. This procedure eliminated any potential variables caused by the cleaning procedures. After exhaustion, the resins were virtually 100% in the calcium form.
Each column of calcium form resin was regenerated with 3 successive doses of salt at a 5 pound per cubic foot level. The spent brine and displacement volume of each dose were collected and analyzed for hardness. From this, the available sodium form capacity was calculated. These results were then used to analyze the effectiveness of the various cleaners.
Brief Description of theCleaners and Cleaning Procedures
NOTE: All samples, except "A", were air lanced and backwashed for 30 minutes prior to cleaning.
First series of tests (performed with iron cleaners)
Sample A
This reference sample was not cleaned but was regenerated in the same way as all the others. The results should approximate the field performance of the resin without any cleaning.
Sample B
This sample was treated with an excess of hot hydrochloric acid in order to remove all the iron from the resin. The total amount of iron on the resin can be used to compare the various cleaners with respect to their ability to remove iron. Approximately 21 pounds per cubic foot of 5% HCl was heated to 120°F and passed through the column in 30 minutes.
Sample C
This cleaner is primarily composed of hydrochloric acid and a proprietary organic chemical. Just over 1 void volume of full strength cleaner was poured into a drained column and allowed tosoak overnight.
Sample D
This cleaner is a mixture of sodium hydrosulfite and sodium metabisulfite. These chemicals have traditionally been used to clean iron fouled resin.One pound per cubic foot of cleaner plus 6 pounds per cubic foot of salt were dissolved in water and passed through the column in 30 minutes.
Samples E and F
Samples E and F were cleaned in a 2 step process; first for oil and second for iron. The first cleaner is a mixture of surfactants and apolar solvent.
One-quarter pound per cubic foot of cleaner was mixed with 5gallons per cubic foot of wate rand poured into a drained column. After 30 minutes, thecolumn wasdrained to bed leveland allowed to soak for 4 hours.
Sample E
After cleaning for oil, this sample received a second treatment as follows. This cleaner is a mixture of formic acid, a chelant similar to EDTA, and a surfactant. Two pounds per cubic foot of cleaner was dissolved into 10 gallons per cubic foot of water and poured into a drained column. After 30 minutes the column was drained to bed level and allowed to soak for 2 hours
Sample F
After cleaning for oil, this sample received a second treatment as follows. This cleaner is primarily sulfamic acid, plus a corrosion inhibitor and stabilizer.
Two pounds per cubic foot of cleaner was dissolved into 10 gallons per cubic foot of water and poured into a drained column. After 30 minutes, the column was drained to bed level and allowed to soak for 2 hours.
Second Series of Tests (performed with Oil Cleaners)
Sample I
Tide is a household laundry detergent that has often been used to clean oil fouled resin.
One-half pound per cubic foot of cleaner was mixed with 4 gallons per cubic foot of water and poured into a drained column. The column was then air mixed for 1 hour.
Sample II
Sometimes a polar solvent is an aid to a surfactant to dissolve oil. One-half pound per cubic foot of tide plus 8 ounces per cubic foot of rubbing alcohol was mixed with 4 gallons per cubic foot of water and poured into a drained column. The column was then air mixed for 1 hour.
Sample III
See samples E and F. In this test the oil cleaner was not followed by other treatments, in order to evaluate its effectiveness as a single cleaner.
Sample IV
This test was performed at the request of the customer who had sulfurous acid available as a waste product from their process. Five pounds per cubic feet of 5% sulfurous acid was passed through the column in 30 minutes.
Sample V
Since "oil" is generally soluble in caustic, this sample was treated with dilute caustic for oil removal. Following this procedure, the sample was treated with hot hydrochloric acid to remove any precipitants that might have formed.
Approximately 10 pounds per cubic foot of 5% NaOH at room temperature was passed through the column in 30 minutes. Following a displacement rinse, approximately 2 pounds per cubic foot of 5% HCl, heated to 120°F, was passed through the column in 30 minutes.
Sample VI
Sodium hypochlorite (bleach) is not normally used as a resin cleaner but has occasionally been used to clean biologically fouled resin beds.
Approximately 3 pounds per cubic foot of 0.5% NaOCl was poured into a drained column and allowed to soak for 1 hour.
Evaluation of the Results
Sample A
This was the sample that was not cleaned. The resin has reasonably good operating capacity, in spite of its fouled condition. Operating capacities varied from 75% of new resin--at the lowest dose, to 90% of new resin--at the highest dose.
This result is in accordance with the way that most systems react to fouling. Increased chemical doses become necessary to produce satisfactory results. Overall efficiency suffers greatly. Operation at low chemical doses becomes impossible.
Sample B
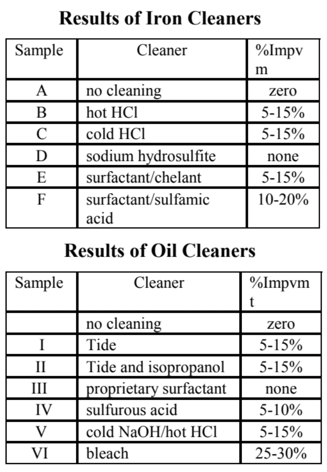
Hot HCl is the cleaner most often recommended by resin manufacturers even though it is seldom practical for field use. The main reason for using this cleaner was to remove the iron from the resin in order to judge the extent of iron fouling. The total iron on the sample was about 0.5 mg of Fe per ml of resin. This is not a particularly high level of iron fouling.
After cleaning, the operating capacity improved somewhat--about 15% at the low dose, and about 5% at the high dose.
Sample C
This cleaner is essentially cold hydrochloric acid. It was used at a much lower dose and at a lower concentration than for Sample B. It was much less effective than hot hydrochloric acid to remove iron. Only 0.04 mg of Fe per ml of ml of resin was removed. However, the improvement in performance was almost as good as that of hot HCl.
Sample D
Sodium hydrosulfite is a traditional cleaner for iron. It removed 0.05 mg of Fe per ml of resin, about 10% of the iron on the resin. After cleaning, the performance was slightly worse than before.
Sample E
Two cleaners were used, the firstcleaner, a surfactant for oil removal, followed by an acid cleaner for scale removal. The net iron removed was 0.05 mg of Fe per ml of resin. Overall improvement in performance was similar to that of the other acid cleaners.
Sample F
Two cleaners were used. The first cleaner was a surfactant for oil removal followed by sulfamic acid, a traditional cleaner for iron.This combination of cleaners removed only 0.02 mg of Fe per ml of resin, yet the performance improvement was very significant, to within 10% of new resin and more than 20% better than the fouled sample that was not cleaned. This combination of cleaners was clearly effective to restore performance, even though very little iron was removed by the resin.
The data generated was submitted to the customer. ResinTech was requested to continue evaluating cleaners. The first group of cleaners were all pre-mixed, commercially available materials, specifically designed for iron removal. The second batch of cleaners were all generically available chemicals designed for oil removal. These samples were not tested for iron removal.
Sample I
Powdered laundry detergents have traditionally been used to clean oil fouled resin. This treatment improved performance by more than 15%.
Sample II
A polar solvent was added to the laundry detergent to try to help improve oil removal. The performance after cleaning improved but was essentially the same as with detergent alone.
Sample III
The oil cleaner from the first batch of samples had not been evaluated separately. It was tested without a second cleaner. There was no change in the resin's performance after cleaning compared to the sample that was not cleaned.
Sample IV
Sulfurous acid produced about the same performance improvement as other acid cleaners somewhere around 10% depending on regenerant dosage.
Sample V
Sodium hydroxide is sometimes effective to remove oil. This cleaner was followed by hot HCl to remove any precipitants formed during the caustic treatment.Overall performance improvement was similar to that found with other acid cleaners.
Sample VI
Bleach is not normally considered as a cleaner, due to its reputation for degrading ion exchange resin.However, the bleach treatment restored performance to virtually the same as new resin. After treatment, there was no measurable difference in the total capacity or moisture content of the resin. Of all the cleaners tested bleach was the most effective.
Conclusions
The original task presented to ResinTech by our customer was to test the effectiveness of various cleaners on their resin. They assumed that the foulant was iron. Iron cleaners are not particularly effective for oil fouled resin. An obvious conclusion here is that a cleaner should be appropriate for the foulant that is causing the problem. A little lab work can save a lot of frustration in the field.
A combination of cleaners, a surfactant followed by an acid cleaner, was much more effective than either type of cleaner alone. Our conclusion is that the first cleaner removed enough of the oil barrier to allow the second cleaner to penetrate the beads.
Why did bleach work so much better than cleaners that are traditionally used to clean oil fouled resin?
We believe that the bleach reacts with oil on the surface of the resin beads, defeating the hydrophobic barrier created between the resin and water. This permits the exchange of ions to occur in amore normal way.
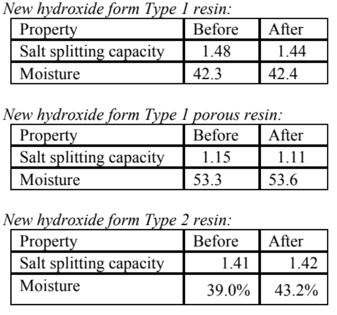
The long term effect of oxidants on ion exchange resins is well documented. Long term exposure to low levels of chlorine causes the resin to lose capacity. This does not mean that short exposure to higher levels are equally damaging. In fact, there is very little effect on the resin with a 1 hour exposure to 0.5% beach, whereas a 5,000 hour exposure to1 ppm of bleach causes a noticeable change in the resin.
The rate of oxidation of the resin depends on several factors such as bleach concentration, temperature, whether or not the resin is the only oxidizable substance for the bleach to attack. The degree of oxidative damage to the resin is cumulative and therefore depends on the total of all oxidizing exposures since oxidation of the resin is an irreversible process.
When the resin is coated or fouled with oxidizable substances such as oil, grease, or organics (organic fouling), these substances will compete with the ion exchange resin to become oxidized by the bleach. In some cases, especially when the resin is coated with oil, these substances will form a protective coating on the resin and protect it from attack by the bleach. While this is happening, the resin is suffering none or very little attack. Even after the oil film around the resin has been broken, the remaining oil continues to compete with the resin for the bleach thus offering continued protection, albeit somewhat reduced. While all this is happening, the bleach is being consumed and its concentration is lowered. Proper selection of the bleach concentration is as important as the time of exposure and frequency of treatments in optimizing the overall performance of a resin including its effectiveness and life. If the bleach concentration is too low, then there is the possibility of not having enough to remove the foulants. Likewise, if the time period is too short, or the temperature too cold, etc., the treatment will be less effective. Figures 3 and 4 show how the rate and cumulative effect of bleach treatments affect an ion exchange resin when done properly.
It is always best to "trial" the procedure in a lab column before attempting treatment of the entire resin bed. A procedure that works well at one installation may not work in another, even though the foulant appears similar. It is unlikely that severely fouled resin will ever again perform like new resin. Cleaning resin almost always causes some damage to the resin and some resin loss.Removing the foulant(s) from the feedwater is almost always abetter alternative than periodic cleaning.
Lab Data & Graphs
Iron Cleaners Evaluated
A. reference -- no cleaning
B. hot hydrochloric acid
C. cold hydrochloric acid
D. sodium hydrosulfite
E. surfactant/chelant
F. surfactant/sulfamic acid
Oil Cleaners Evaluated
A reference--no cleaning
I tide
II tide plus isopropanol
III proprietary surfactant
IV sulfurous acid
V cold sodium hydroxide/hothydrochloric acid
VI sodium hypochlorite
Basic Lab Procedure
1. 100 ml resinin 1-inch column
2. air lance and backwash
3. apply cleaner
4. rinse
5. exhaust resin with calcium chloride
6. regenerate with sodium chloride
7. analyze spent brine for hardness
Conclusions
*iron cleaners are not effective oil cleaners
*acid cleaners are somewhat effective
*generic laundry detergents are effective
*bleach is very effective
*bleach does not significantly damage resin with short exposures
Cation Resin Exposure to Bleach (0.5% NaOCl for one hour)

![]()
Anion Resin Exposure to Bleach (0.5% NaOCl for 1½ hour)

Anion Resin Exposure to Bleach (0.5% NaOcl for 1½ hour)