Selective Silica Removal
By Peter Meyers
Introduction
The contaminants in power plant primary water systems have been a longterm concern and something of a holy grail to water treatment professionals. Typical contaminants include trace metals such as aluminum, calcium, and magnesium, in addition to elevated concentrations of dissolved and colloidal silica. The metals are removed (reasonably well)through standard commercially available demineralizer packages. However, resins currently on the market have had very little effect on the removal or reduction of primary borated silica concentrations.
Silica is found in almost all water supplies, is moderately soluble and there is no convenient method of selectively removing it, without removing most of the other ions along with it. These water-borne impurities contribute to the formation of crud deposits on nuclear fuel elements. Incorporation of these impurities into this crud can cause a significant barrier to the heat transfer and densification of the crud at the clad surface.
The sources of contamination are the large primary water supply inventories such as the Spent Fuel Pool (SFP), the Refueling Water Storage Tanks (RWST) and the boric acid tanks. In addition, silica is introduced to the Reactor Coolant System (RCS) through any necessary makeup water.
Up until now, silica concentrations are reduced by extensive time consuming feed and bleed operations and/or expensive membrane processes. Current processes either result in a vast amount of water used for process and discharge or with a concentrated waste stream that may still needs to be treated for release.Both means of processing also require boron makeup to inventories. There currently is no method to remove silica without also reducing the boron concentration.
As mentioned, silica is introduced from makeup water or from any silica bearing equipment / material containing that is exposed to the water. However, the main contributor to elevated levels in power plant primary waters is due to the silica leaching from the borosilicate fuel racks. This evolution has shown to cause an increased concentration exceeding limits set by system suppliers. Not only are select primary water chemistry specifications out of spec, but the increased levels may jeopardize any existing reactor fuel warranties.
The hybrid exchangers BSM-50 and ASM-125 are already in use on borated waters to remove traces of antimony and other activated corrosion products. In addition to their affinity for contaminants such as antimony and tin, the hybrid ion exchangers have substantial capacity for silica and will remove silica preferentially from neutral waters. Although the removal is not complete, the process is able to reduce silica levels from borated waters, without removing the boron or adding any other unwanted contaminants. This resin can be used in current in-plant system allowing plants to remain within chemistry specs during for normal power and refueling operations.
This paper provides background information on the development and testing of this resin. It presents some bench scale test results showing how the hybrids perform in simulated radwaste and spent fuel pool system waters under process operations scaled to actual power plant system designs.
The hybrids
ResinTech developed three hybrid exchangers capable of pulling out silica. Each hybrid is made from a base type I strong base anion resin with a hydrated iron oxide hybrid adsorbent monatomically inserted into the gel phase of the polymer (separate from the ion exchange groups). The three products differ only in the ionic form.
1.ASM-125 is supplied in the chloride form
2.ASM-125-OH is supplied in the hydroxide form*
3.BSM-50 is supplied in the borate form
Both the hydroxide and borate forms have very low chloride content.
*The hydroxide form is slightly unstable and has limited shelf life due to increase in particulate iron oxide sloughage as the resin ages.The hybrid works by first attracting and then exchanging contaminants into the resin beads. Once inside the contaminant comes in close contact with the hybrid material and precipitates, thus effectively trapping the contaminant and locking it inside the resin. The marriage between the ion exchange resin and the adsorbent produces a hybrid with unique properties not found in either parent.
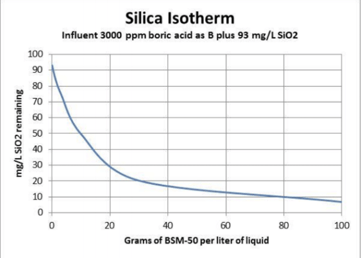
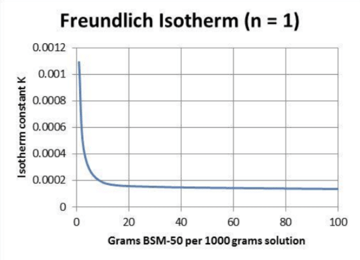
Silica removal seems to follow standard adsorption isotherms. The following chart shows the isotherm for silica removal from boric acid.
Simulated fuel pool
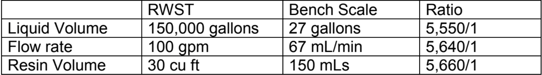
A series of bench scale tests was performed to demonstrate the efficacy, determine the amount of hybrid material needed and to verify that boron was not removed and iron did not leach.
The first test was a simulated fuel pool with 2000 ppm boric acid (as B) plus approx. 110 mg/L of silica (as SiO2)
Initial scaling was as follows

The resin columns were configured such that the pump pulled suction from the bottom of the tank and the effluent was returned to the top of the tank. Each morning the contents in the tank was briefly stirred, then sampled. Each afternoon the column effluent was sampled. Samples were analyzed for pH, boron content, silicon, and iron (iron was spot checked).
Before discussing the silica results it is perhaps worth considering the possible change in composition of the fuel pool itself. In general the effect was minimal. As might be expected, the hydroxide form resins removed boron for a short period of time, then equilibrated as the resin converted into the borate form. After that, there was no significant change over time.
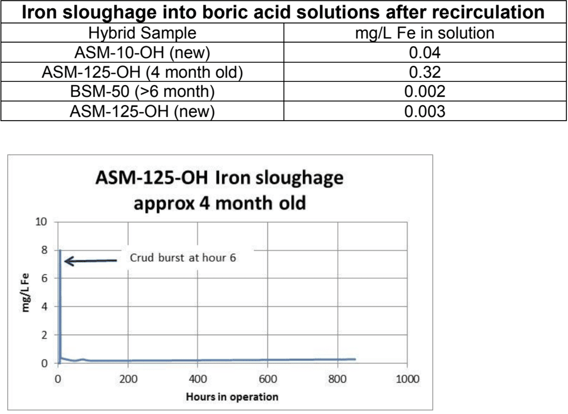
The sample of ASM-125-OH was approx. 4 months old and had started to show a crust of iron on its surface. As the resin converted into the borate form it shrank. The crust loosened and came off the surface of the resin, resulting in a brief (less than 5 minute duration) crud burst of particulate iron. This led to an increase in iron concentration in the entire solution and imparted a somewhat cloudy appearance. Iron was gradually filtered out by the resin, however, every time the flow rate stopped or changed suddenly, a brief but smaller crud burst occurred. Total iron in the solution increased to approx. 0.3mg/L due to the particulate sloughage.
The second column, with freshly regenerated hydroxide form hybrid resin did not exhibit this iron sloughage, nor did the later tests with older borate form hybrid or with reasonably fresh (less than 3 month old) hydroxide form hybrid.
The hybrid used in the second column was slightly different than ASM-125-OH and was prepared from ASM-10-HP. This product uses a type II strong base anion resin rather than the type I strong base anion resin used in ASM-125-OH. The silica removal by the Type II hybrid was not quite as good as from the Type I hybrid.
New hydroxide form hybrid
Form month old hydroxide form hybrid
Two year old hydroxide form hybrid
Except for the crud burst from the aged ASM-125-OH, there was very little change over time in the solution composition, except for a very brief time while the hydroxide form resin was converting into the borate form. The following charts show the change in boron and pH over time.

Silica Reduction
Silica removal was quite rapid for the first 300 to 400 bed volumes, then slowed. The effluent from the resin column was directed back into the same tank used to feed the column. This resulted in a steady decrease in the silica concentration in the feed.

The first treatment reduced the silica from 110 ppm to approx. 60 ppm, the second from 60 ppm to 20 ppm. Although silica removal was not complete, the two treatments resulted in more than 80% reduction of the silica in the borated water, without changing the overall chemistry of the solution.
ASM-125-OH (made with a type I anion resin parent) demonstrated better silica removal than ASM-10-OH (made with a type II anion resin parent). ASM-125-OH also exhibited lower TOC leaching.
The spent resin was digested and analyzed for trace metals.
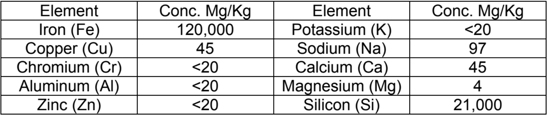
The iron represents the hybrid adsorbent inside the resin polymer. Silica is absorbed during the treatment process. Other metals might be from the ferric chloride and salt used in the manufacturing process. However, the very high iron content in the matrix makes the analysis of other trace metals quite difficult.
Second test
The second test was done with somewhat higher boron content (3000 ppm as B) and lower silica (16 ppm as SiO2). Solution volumes and flow rates were the same as in the first test, however the resin volume was reduced to150 mLs.Three resins were trialed, ASM-125-OH, ASM-10-OH, and BSM-50. BSM-50 is similar to ASM-125 but is provided in the borate form rather than the hydroxide form. The two hydroxide form resins were freshly made and none of the columns leached iron at measurable levels.
The test was scaled to mimic a RW tank
Silica Results
Silica removal by the hydroxide form and borate form hybrids made with type I anion resin were similar. BSM-50 produced the lowest residual silica in solution but also started with somewhat lower silica in solution. Silica removal by hydroxide form ASM-10-OH (with a type II anion parent) was not as good. It took three (smaller) treatments to approach the goal of 2mg/L of silica remaining in solution.
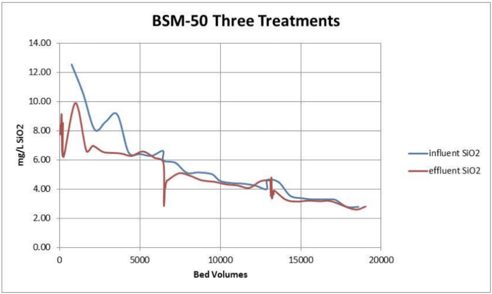
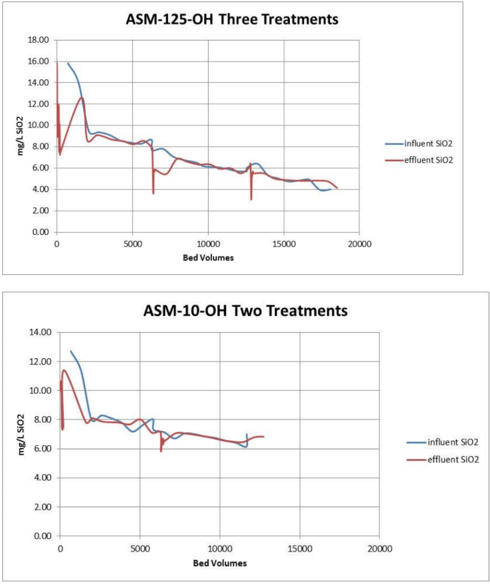
In all three tests there was no measurable iron sloughage. Iron levels in the borated recirculating water remained at less than 10 ppb throughout the test.
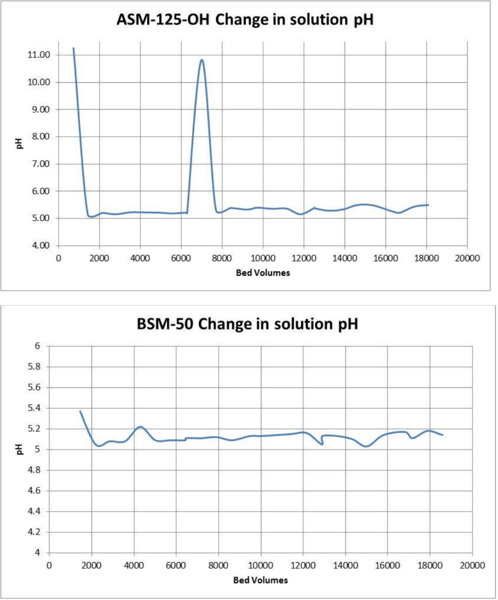
Boron levels and pH quickly stabilized. The hydroxide form resins caused a significant spike in pH initially compared to the borated form BSM-50.
Summary
All three resin candidates were able to remove silica from the borated waters. The borate form resin BSM-50 performed the best, the hydroxide form hybrid made with a type II anion resin did not work quite as well as the hybrids made from a type I anion parent. The borate form resin did not remove boron from the solution and did not cause a pH spike. Both hydroxide form resin did initially remove some of the boron and also significantly increased the effluent pH for a brief time before reaching equilibrium with the inlet solution.
The lack of good mixing in the solution tanks made the process slower than expected. Unfortunately, this is probably the reality in most fuel pools as their original design did not envision the need for this type of treatment.
In all cases, breaking the resin volume up into several increments appears to reduce the overall resin volume required. However the smaller resin volume must be weighed against the longer time needed to reduce the silica.
The hydroxide form hybrids are slightly unstable with respect to iron sloughage. Freshly made hybrid does not release iron but as the resin ages iron accumulates externally on the surface of the beads and can then break off in a crud storm that causes a spike of several ppm iron in the column effluent. Although some iron is picked back up by filtration, the result is variable and inconsistent. The borate form hybrid is stable and does not exhibit any iron sloughage, even after long periods of storage.
Overall, the hybrid anion exchangers were able to meet the research objective of reducing silica from more than 100 ppm to close to 2 ppm without release or iron or other ionic contaminants.














