In this article, we hope to explain some of the difficulties in the measurement of the pH of pure water.
DEFINITION
pH is the measurement of hydrogen ion concentration in a solution. The equation for pH is
pH=-log [H+], the concentration of hydrogen ions can be calculated from the pH. pH is measured on a scale of zero to fourteen, the lower the value the more acidic the sample or the higher concentration of hydrogen ions. Conversely, the higher the pH the more basic the sample or lower concentration of hydrogen ions. A pH of 7 is neutral and has an equal amount of hydrogen ions and hydroxide ions.
The counterpart of pH is pOH. pOH is the determination of hydroxide in solution, using the equation: pOH= -log [OH-]. The pOH scale is the inverse of the pH scale. The lower the pOH the more basic the solution and the higher the pOH the more acidic the solution on a scale of 0-14.
Why is pH used more commonly than pOH? Simply explained pH is easier to say and is more common place in the market. The addition of pH and pOH equals the water auto-ionization constant, pKw.
pH + pOH = pKw=14, at room temperature (25°C)
pH AND CONDUCTIVITY RELATIONSHIP
When measuring the pKw of pure water, the conductivity will be 0.055 μS/cm or 18.18 megaohms. Carbon dioxide, bicarbonates, and carbonates all increase the alkalinity and conductivity of water. Alkalinity and pH go hand in hand. Alkalinity works as a buffer for acidic waters to neutralize the acid and increase the pH. Alkalinity is an excellent buffer and withstands large pH changes in water.
The most common two alkali compounds are sodium bicarbonate (baking soda) and sodium carbonate (soda ash). These two compounds release carbon dioxide in the presence of acid
and raise the pH. The addition of sodium bicarbonate in water will increase the pH to a
maximum of 8.3.
pH MEASUREMENT THEORY
pH measurement is the change in voltage versus a change in pH. Detection is achieved with an electrochemical cell, often called a pH probe. The cell consists of an indicating electrode whose potential is directly proportional to pH, a reference electrode whose potential is independent of pH, and the liquid to be measured. The overall voltage of the cell depends on the pH of the sample. Different indicating electrodes have slightly different responses to pH, the measuring system must be calibrated before use and frequently thereafter. Probes are designed to produce a potential of 0 mv at a pH of 7.0, as the potential increases the pH decreases and vice-versa.
The first step of pH measurement is calibration. The system is calibrated by placing the pH probe in solutions of known pH and measuring the voltage of the cell. Cell voltage is a linear function of pH, so only two calibration points are needed. The final step in the measurement of pH is to place the probe in the sample, measure the voltage, and determine the pH from the calibration data. It is apparent that the practical determination of pH requires standard solutions of known pH. The standard solutions are called buffers, and the pH values assigned to them define the pH scale. The equation (slope) implies that pH is a measure of concentration. In fact, pH is really a measure of ion activity. Concentration and activity are not the same, but they are related.

MEASUREMENT/PITFALLS OF PH IN DEIONIZED WATER
Deionized water has very low conductivity, which means it is high in resistance because all the conductive components have been removed. These conducive components are critical to a stable pH measurement. Low conductive solutions are susceptible to contamination (CO2 Effect), static chargers and temperature effects making the accurate pH measurement very difficult. Probes cannot accurately measure water that do not conduct electricity.
CO2 EFFECT
The pH of pure (18.18 megaohms) water is always 7.0, but water does not stay pure for long. When pure water is in contact with the atmosphere the CO2 in the air quickly dissolves into pure water and forms carbonic acid.
H20 + CO2 (g) = H2CO3
Stored pure water can have a pH as low as 5.3. The longer it sits, the lower the pH. The pH of pure water in open air changes within seconds. This is why it is difficult to measure the pH of high purity de-ionized water in the laboratory.
STATIC CHARGES
Pure water is a poor electrical conductor it creates a static charge when flowing past
non-conducting materials of the pH probe. These static charges, called streaming or friction potentials, are comparable to rubbing a glass rod, e.g. glass electrode, with a wool cloth, e.g. the water. The static charge will create stray currents resulting in erratic pH readings. The generated “streaming potentials” in the solution may cause large errors, or at least excessive noise in the readings. An expensive low impedance, well- shielded and grounded electrode can lower these errors. The high resistance water also increases the measurement loop’s sensitivity to surrounding electrical noise sources.
TEMPERATURE EFFECTS
There are two major temperature effects that must be addressed in order to establish a truly accurate representation of pH in high purity water. The standard automatic temperature compensator only corrects for one of these, often referred to as electrode correction. Simply stated, as the glass electrode increases in temperature, its output voltage increases, even though the actual pH of the measured solution may remain the same. The effect is minimal at or near a pH of 7 and increases linearly above and below a pH of 7.
The second temperature effect is known as the equilibrium or dissociation constant correction. While this effect is usually much smaller in magnitude, it can become significant. All solutions respond to changes in temperature in a specific way (dissociation constant). Depending on the solution, the response may be related to changes in pH or conductivity. The dissociation constant of pure water is 0.172 pH/10°C. This means that at 50°C, pure water has a pH of 6.61, while at 23°C it will have a value of 7.04 pH. The amount of temperature change involved and the critical nature of the measurement dictate if this effect must be compensated for or not.
REMEDY TO PURE WATER PH MEASUREMENT
The measurement of pure water pH requires the addition of a buffer to freshly produced pure water. The buffer suggested is a saturated solution of potassium chloride. The potassium chloride solution should be made by dissolving as much potassium chloride as you can into
50-75mls of pure water. You then add 4 drops of concentrated potassium chloride in a clean empty beaker, add 50-100 ml’s of freshly made pure water to that and then measure pH.
The potassium chloride increases response time, does not shift pH and stops the CO2 from forming carbonic acid. If all goes well, the pH should be 7.0. Even this remedy has a pitfall as
the Potassium Chloride must be of high purity, otherwise the contaminants may affect the
pH measurement.

* The measurement of pH in Type I, II, and III reagent waters has been eliminated from this specification because these grades of water do not contain constituents in sufficient quantity to significantly alter the pH.
EXPERIMENTAL DATA
An experiment was conducted under controlled conditions to measure the pH of deionized
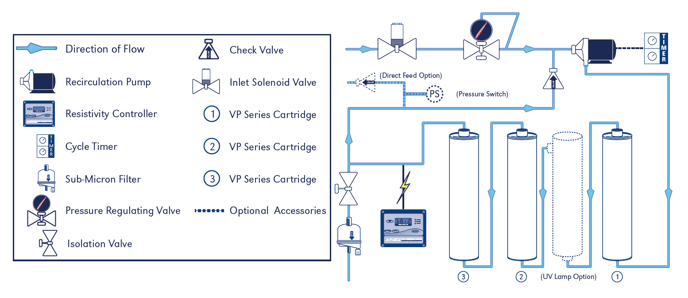
water. An ultra high purity water system (Figure 1) was configured with in-line pH and
Resistivity probes.
The CLïR 3000 system is a compact ultra high purity water purification system designed to
make ASTM Type I water. It consists of a recirculation pump and 3 deionizing filters. The water
is continually polished in a closed loop system with no exposure to the atmosphere. Water measurements are taken inline as the water flows through the Cell Well containing pH and Resistivity probes. The results are output on external displays where the data was gathered. Grab samples were also taken. The pH and resistivity results were recorded immediately as well as over time as the sample was exposed to the air.
The CLïR 3000 water system contains 3 high purity mixed bed filters in series. High purity mixed bed ion exchange resin is a physical mixture of strong acid cation resin in the hydrogen form (H) and strong base anion resin in the hydroxide form (OH). Mixed bed ion exchange resins are widely known to produce ultra high purity, 18.18 megaohm water.
FIGURE 1 – CLïR 3000 HIGH PURITY LOOP DIAGRAM
The experimental data collected is displayed in Table B. In every experiment the resistivity of the water was very high up to 18.02 megaohms. Theoretically, we know that the pH of this water is 7.0. However, the pH as monitored in-line was ranged from 4.79 to 5.78.
The grab samples were higher than the in-Line pH measurements but not consistent. The grab sample pH continued to drop as the sample was exposed to the air. The longer the sample was exposed to air, the lower the pH continued to drop. The pH drop is due to the intrusion of carbon dioxide into the water. Carbon dioxide (CO2 (g)) can results in as much as 2 pH units.
TABLE B – EXPERIMENTAL DATA
CONCLUSION
Water is often referred to as “The Universal Solvent”. This is especially true of highly purified,
de-ionized or demineralized water. The results of the experiment show that while the theoretical pH of high purity water is 7.0, the inline pH measurements are neither consistent nor accurate. Carbon dioxide (CO2 (g)) in our atmosphere readily dissolves into high purity water. This causes the resistivity and the pH to drop over time from exposure to the air.
The pH measurement depends on voltage feedback to the probe and is temperature dependent. The solution being analyzed must have characteristics suitable to the pH measurement. Deionized water is not suitable for pH measurement with conventional pH meters.
The industry recognizes the problems associated with measuring the pH of high purity water. ASTM Specifications (see Table A) for high purity water go so far as to exclude the measurement for water quality greater than 0.2 megaohms.

